Medical Cannabis May Help Treat Cachexia
- The Three Stages of Cachexia and Their Symptoms
- Treatments for Cachexia
- Treating Cachexia with Cannabinoids
- Recent Research on Cachexia and Medical Marijuana
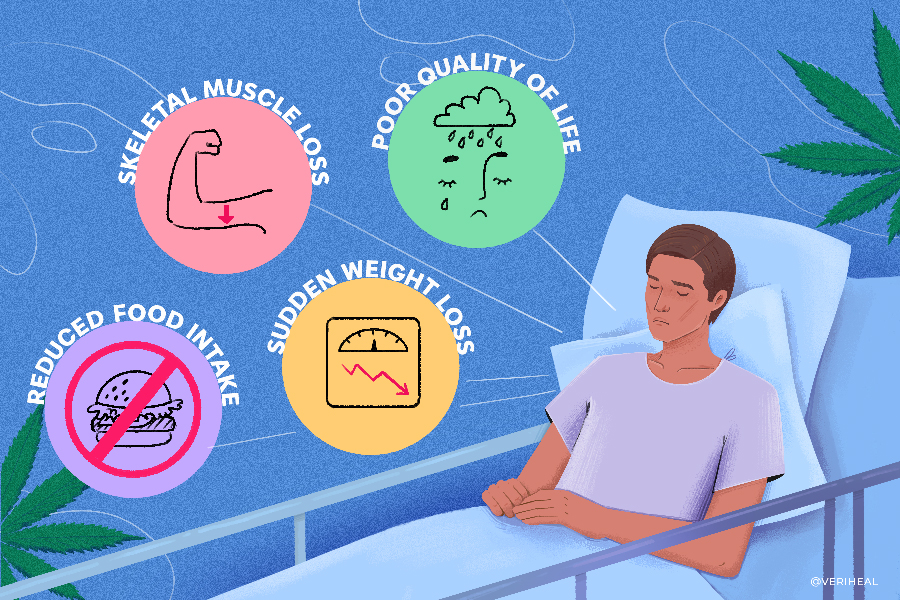
In the late stages of severe and fatal conditions, the body can enter cachexia, which is an extreme loss of weight and body fat. Also called “wasting syndrome,” cachexia is unlike other eating disorders, including anorexia nervosa since it’s metabolic due to chronic conditions including HIV/AIDS, COPD, cancer, congestive heart failure, multiple sclerosis and kidney disease. Symptoms of cachexia include a loss of appetite, severe weight loss, muscle wasting, anemia, and fatigue or weakness.
Though the condition, most prominent in patients in the last stages of life, could be fatal specifically for cancer sufferers, lots of hope in medical marijuana alleviating symptoms has surfaced since the endocannabinoid receptors in the Endocannabinoid System (ECS) balance appetite and control pain, which has the potential to improve patients’ quality of life.
The Three Stages of Cachexia and Their Symptoms
There are three stages of cachexia—precachexia, cachexia, and refractory cachexia (19). Each of these different stages of cachexia presents with different symptoms. Here is a breakdown of each stage of cachexia and its related symptoms.
Precachexia
Precachexia is characterized by a lack of hunger, altered metabolism, and inflammation, which sets in when a patient loses up to 5% of their body weight directly related to any of the aforementioned conditions.
Cachexia
Cachexia is characterized by a patient losing more than 5% percent of their body weight in less than a year due to one of the chronic illnesses. Patients will show signs including lack of appetite, fatigue, inflammation, and muscle weakness.
Refractory cachexia
Refractory cachexia is specifically related to cancer patients who no longer respond to treatment, losing both muscle and ability to function. “The refractory cachexia phase is determined by the patient’s underlying disease and overall condition; diagnosis of this stage requires a low WHO performance status score and a survival period of fewer than 3 months. (13).”
Treatments for Cachexia
While there is no singular, universally effective treatment that has been developed to prevent, hamper, or reverse cachexia progression. There are several ways medical professionals address cachexia in patients despite the lack of efficacious treatments. These therapies include stimulating the patient’s appetite via pharmaceuticals such as megestrol acetate (Megace) and Medroxyprogesterone Acetate (MPA) which are hormone analogs with unknown mechanisms of action. Fewer than 30% of patients taking Megace experience short-term appetite improvements (16). Other medications are used to reduce inflammation like corticosteroids, however, these carry many unfavorable long-term side effects and so are reserved for late-stage illness. Other interventions include changes in diet, and Dronabinol—the active ingredient in the pharmaceutical Marinol—a synthetic cannabinoid modeled after THC. Marinol (dronabinol) was first FDA-approved for the treatment of chemotherapy-induced nausea and vomiting in patients that don’t respond to other therapies in 1985, as well as anorexia related to weight loss in patients with AIDS in 1992. (7)
Treating Cachexia with Cannabinoids
A 2015 journal Nature confirms medical cannabis can stimulate the appetite in the brains of mice. The preclinical study concluded that increased cannabinoid receptor 1 stimulates appetite. In 2014 the journal Innovations in Clinical Neuroscience previously confirmed the same, explicitly noting acute use of cannabis can produce an effect called the “munchies,” which is one of the better-known effects of cannabis (14).”
“In support of these acute appetite-enhancing effects, several authorities report that marijuana may increase body mass index in patients suffering from human immunodeficiency virus and cancer,” according to the study (14).
While there is no conclusive evidence that medical cannabis can treat cachexia, what is undeniable is that it can successfully increase appetite and treat inflammation, another hallmark of cachexia.
Medical Marijuana and Appetite Stimulation
According to the 1999 Institute of Medicine publication Marijuana and Medicine: Assessing the Science Base, “The profile of cannabinoid drug effects suggests that they are promising for treating wasting syndrome in AIDS patients. Nausea, appetite loss, pain, and anxiety are all afflictions of wasting, and all can be mitigated by marijuana. Although some medications are more effective than marijuana for these problems, they are not equally effective in all patients (6).”
A 2018 study of the use of oral dronabinol in HIV/AIDs patients with anorexia managing severe weight loss which, “revealed a positive effect on weight gain and led to several studies that were done with cancer patients. Those studies did not meet their primary endpoint. However, dronabinol was associated with improved taste, smell, and food enjoyment (1).” A 2015 systematic review published in JAMA found low-quality evidence that supports the use of cannabinoids for weight gain in HIV-related cachexia (20).
It’s clear that delta-9 THC has the potential to increase food intake and promote weight gain in patients, as well as possibly adding the benefit of reducing nausea and vomiting associated with chemotherapy. “It is therefore unsurprising that clinicians were keen to assess the utility of cannabinoid treatments in relation to clinical syndromes involving appetite or weight loss. A few, somewhat limited, studies have been conducted with delta 9-THC to examine the drug’s capacity to ameliorate low appetite and wasting in clinical populations with cancer cachexia or HIV. One of the earliest trials, by Regelson et al. (1976), found that oral 9-THC doses of up to 15 mg/d stimulated appetite and produced significant weight gain in advanced cancer patients (8).”
Medical Cannabis and Inflammation
The inflammatory process is the body’s response to a harmful stimulus that takes place to restore homeostasis in the body. Anti-inflammatory drugs (NSAIDs) although effective have a range of adverse effects attributed to them. “In this sense, herbal medicine and derivatives have gained more attention because of their effectiveness and safety, showing the importance of medicinal plants, especially the Cannabis genus and the cannabinoid derivatives (12).” With more research being conducted, cannabinoids are becoming known as potential novel anti-inflammatory drugs (11).
In a review of six studies from 1980 to 2019 that look at cannabis and inflammatory mediators, researchers concluded that cannabis seemed to lower levels of inflammation in healthy subjects. However, it did not have a significant impact on patients with highly dysfunctional inflammatory activation (10).
In a more recent review of the current research published in 2021, “The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies,” researchers found that “In 22 studies where cannabidiol (CBD), cannabigerol (CBG), or CBD in combination with delta-9-tetrahydrocannabinol (THC) were administered, a reduction in the levels of at least one inflammatory cytokine was observed, and in 24 studies, some improvements in disease or disability were apparent (5).” The study also suggested that CBD and CBG be focused on in future clinical studies for potentially more anti-inflammatory potential than THC alone.
Recent Research on Cachexia and Medical Marijuana
In 2006, the first phase III, double-blind, clinical trial with patients with cancer-related cachexia compared the effects of cannabinoids with a placebo and standardized cannabis extract or “CE”. Researchers “found no differences between the three groups over 6 weeks of treatment for the primary endpoints of appetite and QOL, for cannabinoid-related toxicity, or for secondary endpoints such as mood or nausea (15).” Limitations to this study include a lack of experimentation with adding cannabidiol (CBD) to temper adverse effects associated with delta-9 THC, as well as most likely using too low of a dose—participants took 2.5mg twice daily for only 6 weeks—to observe significant impacts.
A pilot study published in 2019 aimed to evaluate the effect of dosage-controlled cannabis capsules on cancer anorexia-cachexia syndrome (CACS) and weight loss in late-stage cancer patients. Preliminary findings in this study exhibited “a weight increase of ≥10% for 3 patients (50% of those patients who completed the study). The remaining patients had stable weights. Also, all patients who were involved in the study for 4.5 months reported an increase in appetite, as did 83% of the patients who completed the study. For 50% of the patients who completed the study, there were reports of pain reduction and sleep improvement. Additional results showed a significant decrease of appetite loss complaints among 83% of the patients who completed the study (1).”
While small studies like these show promising results, a 2021 systematic review in Journal of Cannabis and Cannabinoid Research looking at a few studies like these acknowledges that research on cancer-related cachexia and cannabis still needs more robust data before recommendations can be made.
Although research on CACS and cachexia is still in its infancy, there is encouraging evidence that medical marijuana could potentially be part of treatment for patients losing muscle mass and body weight through increasing appetite.
Note: The content on this page is for informational purposes only and is not intended to be professional medical advice. Do not attempt to self-diagnose or prescribe treatment based on the information provided. Always consult a physician before making any decision on the treatment of a medical condition.
1. Bar-Sela, G., Zalman, D., Semenysty, V., & Ballan, E. (2019). The effects of dosage-controlled cannabis capsules on cancer-related cachexia and anorexia syndrome in Advanced cancer patients: Pilot study. Integrative Cancer Therapies, 18, 153473541988149. https://journals.sagepub.com/doi/full/10.1177/1534735419881498
2. CM;, K. T. C. W. (2001, June). Endogenous cannabinoids and appetite. Nutrition research reviews. Retrieved April 18, 2022, from https://pubmed.ncbi.nlm.nih.gov/19087417/
3. Gorter, R. W. (1999). Cancer cachexia and cannabinoids. Complementary Medicine Research, 6(3), 21–22. https://www.karger.com/Article/Abstract/57152
4. Hammond, S., Erridge, S., Mangal, N., Pacchetti, B., & Sodergren, M. H. (2021). The effect of cannabis-based medicine in the treatment of Cachexia: A systematic review and meta-analysis. Cannabis and Cannabinoid Research, 6(6), 474–487. https://pubmed.ncbi.nlm.nih.gov/34664988/
5. Henshaw, F. R., Dewsbury, L. S., Lim, C. K., & Steiner, G. Z. (2021). The effects of cannabinoids on pro- and anti-inflammatory cytokines: A systematic review of in vivo studies. Cannabis and Cannabinoid Research, 6(3), 177–195. https://pubmed.ncbi.nlm.nih.gov/33998900/
6. Institute of Medicine (US); Joy JE, Watson SJ Jr., Benson JA Jr., editors. Marijuana and Medicine: Assessing the Science Base. Washington (DC): National Academies Press (US); 1999. 4, The Medical Value of Marijuana and Related Substances. Available from: https://www.ncbi.nlm.nih.gov/books/NBK230711/
7. Institute of Medicine (US); Joy JE, Watson SJ Jr., Benson JA Jr., editors. Marijuana and Medicine: Assessing the Science Base. Washington (DC): National Academies Press (US); 1999. 5, Development of Cannabinoid Drugs. Available from: https://www.ncbi.nlm.nih.gov/books/NBK230708/
8. Kirkham, T. C., & Williams, C. M. (2001). Endogenous cannabinoids and appetite. Nutrition research reviews, 14(1), 65–86. https://pubmed.ncbi.nlm.nih.gov/19087417/
9. Koch, M., Varela, L., Kim, J. G., Kim, J. D., Hernández-Nuño, F., Simonds, S. E., Castorena, C. M., Vianna, C. R., Elmquist, J. K., Morozov, Y. M., Rakic, P., Bechmann, I., Cowley, M. A., Szigeti-Buck, K., Dietrich, M. O., Gao, X.-B., Diano, S., & Horvath, T. L. (2015). Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature, 519(7541), 45–50. https://pubmed.ncbi.nlm.nih.gov/25707796/
10. Lima, M. G., Tardelli, V. S., Brietzke, E., & Fidalgo, T. M. (2020). Cannabis and inflammatory mediators. European Addiction Research, 27(1), 16–24. https://pubmed.ncbi.nlm.nih.gov/32726782/
11. Nagarkatti, P., Pandey, R., Rieder, S. A., Hegde, V. L., & Nagarkatti, M. (2009). Cannabinoids as novel anti-inflammatory drugs. Future Medicinal Chemistry, 1(7), 1333–1349. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2828614/
12. Nascimento Menezes, P. M., Valença Pereira, E. C., Gomes da Cruz Silva, M. E., da Silva, B. A., de Souza Duarte Filho, L. A., de Lima Araújo, T. C., Bezerra Lima, K. S., Silva, F. S., & Rolim, L. A. (2019). Cannabis and cannabinoids on treatment of inflammation: A patent review. Recent Patents on Biotechnology, 13(4), 256–267. https://pubmed.ncbi.nlm.nih.gov/31237222/
13. Ni, J., & Zhang, L. (2020). Cancer cachexia: Definition, staging, and emerging treatments. Cancer Management and Research, Volume 12, 5597–5605. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7358070/#:~:text=Cancer%20cachexia%20is%20divided%20into,and%20stage%20of%20the%20tumor
14. Sansone, R. A., & Sansone, L. A. (2014, July). Marijuana and body weight. Innovations in clinical neuroscience. Retrieved May 2, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4204468/
15. Strasser, F., Luftner, D., Possinger, K., Ernst, G., Ruhstaller, T., Meissner, W., Ko, Y.-D., Schnelle, M., Reif, M., & Cerny, T. (2006). Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the cannabis-in-cachexia-study-group. Journal of Clinical Oncology, 24(21), 3394–3400. https://ascopubs.org/doi/10.1200/JCO.2005.05.1847
16. Tazi, E. M., & Errihani, H. (2010). Treatment of cachexia in oncology. Indian Journal of Palliative Care, 16(3), 136. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3012235/
17. Wang, J., Wang, Y., Tong, M., Pan, H., & Li, D. (2019). Medical cannabinoids for cancer cachexia: A systematic review and meta-analysis. BioMed Research International, 2019, 1–6. https://www.hindawi.com/journals/bmri/2019/2864384/
18. Wang, J., Wang, Y., Tong, M., Pan, H., & Li, D. (2019). New Prospect for cancer cachexia: Medical cannabinoid. Journal of Cancer, 10(3), 716–720. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6360413/
19. Watson, S. (2018, September 18). Cachexia: Definition, treatment, and relation to cancer. Healthline. Retrieved May 2, 2022, from https://www.healthline.com/health/cachexia
20. Whiting, P. F., Wolff, R. F., Deshpande, S., Di Nisio, M., Duffy, S., Hernandez, A. V., Keurentjes, J. C., Lang, S., Misso, K., Ryder, S., Schmidlkofer, S., Westwood, M., & Kleijnen, J. (2015). Cannabinoids for medical use. JAMA, 313(24), 2456. https://jamanetwork.com/journals/jama/fullarticle/2338251